-
Topics
subnavigation
Radioactivity and radiation
Radioactivity is a term for the property of certain atomic nuclei to transform themselves into other nuclei without external influence. During this process, energetic radiation (alpha, beta, gamma or neutron radiation) is emitted. There are both natural radionuclides and artificial radionuclides generated by nuclear processes.
The process of nuclear transformation is usually referred to as nuclear decay, the radiation emitted is referred to as ionising radiation. It has the property to trigger ionisation processes in atoms and molecules when penetrating substances. Therefore, the term "radioactive" radiation, which is frequently used colloquially, is not scientifically correct. Rather, the atoms are "radioactive", which are also termed radionuclides in their entirety.
At the end of the decay chain there are stable atoms
The atomic nucleus that has generated after the transformation can again be radioactive and decay further. The final products of these decay chains are stable atoms that are no longer radioactive.
Different types of radiation
The following types of radiation can occur during nuclear disintegration:
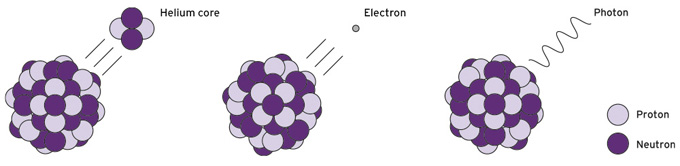
![]() Alpha Beta and Gamma radiation
Alpha Beta and Gamma radiation
Alpha radiationshow / hide
Alpha radiation is particle radiation consisting of two protons and two neutrons. Hence, an alpha particle is a nucleus of the element helium. Alpha particles are absorbed very quickly by matter (by air and water, for example) and for this reason have only a very short range (a few centimetres in air; less than a millimetre in water). They can already be shielded by a sheet of paper.
With external exposure, alpha radiation can only penetrate the outer layers of human skin. Inhaling or ingesting alpha-ray emitters – that is, radioactive substances that emit alpha particles during decay – into the body (incorporation) by air or food can lead to a considerable radiation exposure.
As alpha particles lose their energy within a very short distance, they cause especially severe damage to tissue.
A typical and important example for the incorporation of alpha-ray emitters is the intake of radon and its progeny via inhaled air.
Beta radiationshow / hide
Beta radiation is particle radiation that occurs when radioactive atomic nuclei emit (negatively charged) electrons or – less often – positrons (positively charged particles with the same mass as electrons) as they decay. Beta radiation is much less easily absorbed by matter than alpha radiation and thus has a longer range: The penetration performance of beta particles ranges from a few centimetres to metres in air, a few millimetres to centimetres in soft tissue and plastic. Beta radiation can be shielded quite easily, for example, by an aluminium sheet a few millimetres thick.
Radioactive particles which emit beta radiation can also lead to a considerable radiation exposure when they are taken up by the body (incorporated) via inhaled air or food intake. In the case of external exposure, beta radiation can also damage body tissue as it can penetrate the body even if not very deeply. However, it loses significantly less energy over a certain distance than alpha radiation. We say that beta radiation has a lower biological effectiveness than alpha radiation.
Neutron radiationshow / hide
Neutron radiation consists of uncharged particles (the neutrons). Neutrons are mainly released during nuclear fission, which is a special type of nuclear transformation. Nuclear fission is characteristic only of heavy atomic nuclei (such as nuclei of the element uranium).
Neutron radiation is barely absorbed by air. Shielding is rather elaborate. Materials with the highest possible hydrogen content (such as paraffin, polyethylene, water) are used to initially slow down the neutrons. The slowed-down (thermal) neutrons have to be captured by an absorber (such as boron or cadmium). The gamma radiation emitted simultaneously has to be shielded by lead.
Especially due to the strong interaction with biological tissue (in particular the contained water molecules) neutron radiation has a high biological effectiveness.
Gamma radiationshow / hide
In the case of gamma radiation, energy is transferred as an electromagnetic wave. Electromagnetic radiation can be described in terms of its frequency or its wavelength. The higher the frequency and the shorter the wavelength, the more energetic the radiation. Gamma radiation is at the high energy end of the "electromagnetic spectrum", at the high frequency or short wavelength end.
Gamma radiation results from the decay of radioactive atoms in the atomic nucleus, often in addition to alpha or beta radiation. It penetrates matter very easily. For this reason shielding is elaborate. Heavy materials such as lead and concrete are used.
Both in the case of external exposure and incorporation, gamma radiation is harmful to living beings as it penetrates deeply into tissue. However, its biological effectiveness is lower than that of alpha radiation for instance, as it transfers less energy to tissue over a certain distance.
X-Radiationshow / hide
X-radiation is electromagnetic radiation. It results i.a. from the decay of radioactive atomic nuclei in the atomic shell.
In addition, it can be generated artificially when high-speed electrons are slowed down at the anode (positively charged electrode) of an x-ray tube. The higher the applied tube voltage at which the electrons in the x-ray tube are accelerated, the shorter the wavelength and the higher the energy of the resulting x-radiation.
When the X-ray machine is turned off, no X-radiation is generated.
How radiation affects the body
Irrespective of whether it is of natural or artificial origin, ionising radiation is directly harmful to the cell as the smallest biological unit. Radiation can modify or destroy cellular components and the DNA. However, cell losses or modifications in the cells do not necessarily mean that there will be damage to health.
State of 2017.03.15

